| Specimen | Serum, Whole blood |
| Sample volume | 100µl |
| Range | 1~200ng/mL |
| Time | 15mins |
| Temperature | 2-30? / 36-86? |
| Shelf life | 18 months |
-
STANDARD F CK-MB FIA is a fluorescent immunoassay for the quantitative measurement of Creatine Kinase Isoenzyme-MB level in human serum and whole blood. This test is to screen and monitor the Acute Myocardial Infarction (AMI).
Requires Standard F Analyzer system -
STANDARD F D-dimer FIA performs quantitative measurement of D-dimer level in plasma and whole blood samples using fluorescence immunochromatography. Through the STANDARD F Analyzer, test results with high sensitivity and specificity can be obtained
Requires Standard F Analyzer systemSpecimen Plasma, Whole blood Sample volume 10µl Measurement Range 25-5,000mg/mL Time 7mins Temperature 2-30? / 36-86? Shelf life 18 months -
STANDARD F hs-CRP FIA performs quantitative measurement of high-sensitivity C-Reactive Protein (hs-CRP) in serum, plasma and whole blood samples using fluorescence immunochromatography. Through the STANDARD F Analyzer, test results with high sensitivity and specificity can be obtained.
Requires Standard F Analyzer systemSpecimen Serum, Plasma, Whole blood Sample volume 5µl Range 0.1-15.0mg/L Time 3mins Temperature 2-30? / 36-86? Shelf life 18 months -
STANDARD F NT-proBNP FIA is a quantitative test for the determination of the N-terminal pro brain-type natriuretic peptide (NT-proBNP) level in human serum and whole blood(EDTA).
Requires Standard F Analyzer systemSpecimen Whole blood (EDTA), Serum Sample volume 100µl Measurement Range 0 ~ 25,000pg/mL Time 15mins Temperature 2-30? / 36-86? Shelf life 18 months -
KOREA MFDS Approved
STANDARD F COVID-19 Ag FIA is the fluorescent immunoassay to detect SARS-CoV-2 infection in human nasopharyngeal swab specimen, identifying existence of SARS-CoV-2 viral nucleoprotein antigens.
- Fast results as soon as 15 mins
- Higher sensitivity than rapid test
- Easy to use
- Fluorescent Immunoassay (Europium)
-
This kit is the fluorescent immunoassay to detect the qualitative presumptive detection of specific IgM and IgG to 2019 novel coronavirus (nCoV) in humoral fluid. KOREA MFDS Approved
STANDARD F COVID-19 IgM/IgG Combo FIA is the fluorescent immunoassay to detect the qualitative presumptive detection of specific IgM and IgG to 2019 novel coronavirus (nCoV) in humoral fluid.
- Fast results as soon as 15 mins
- Higher sensitivity than rapid test
- Easy to use
- Fluorescent Immunoassay (Europium)
-
STANDARD F COVID-19 Ag FIA is the fluorescent immunoassay for the qualitative detection of specific nucleoprotein antigens to SARS-CoV-2 present in human nasopharynx. STANDARD F COVID-19 Ag FIA should be used with the STANDARD F Analyzers manufactured by SD BIOSENSOR. This test is for in vitro professional diagnostic use and intended as an aid to early diagnosis of SARS-CoV-2 infection in patient with clinical symptoms with SARS-CoV-2 infection.
-
This fluorescence immunoassay device that can perform qualitative and quantitative analysis on infections, respiratory diseases and chronic diseases supports convenient user interface and LIS/HIS connectivity.
-
This small fluorescence immunoassay device can perform qualitative and quantitative analysis on infections, respiratory diseases and chronic diseases, and it is operable with battery.
-
This kit is the fluorescent immunoassay to detect the qualitative presumptive detection of specific IgM and IgG to 2019 novel coronavirus (nCoV) in humoral fluid.

The STANDARD M Flu/SARS-CoV-2 Real-Time Detection Kit is a real-time reverse transcription PCR assay for the simultaneous detection of Flu A, Flu B and SARS-CoV-2 nucleic acids in human nasopharyngeal swab, oropharyngeal swab, and sputum specimens.
- Single tube reaction for detection of SARS-CoV-2, Flu A and Flu B
- One-step Real-Time RT-PCR
- Provide all reagents required for RT-PCR
- Multiple SARS-CoV-2 target genes: ORF1ab and E gene
- Conserved Flu target region: Flu A (Matrix gene), Flu B (NS1 gene)
- Internal control: RNA process control (from NA extraction)
-
 FDA EUA Approved
FDA EUA Approved  KOREA MFDS Approved
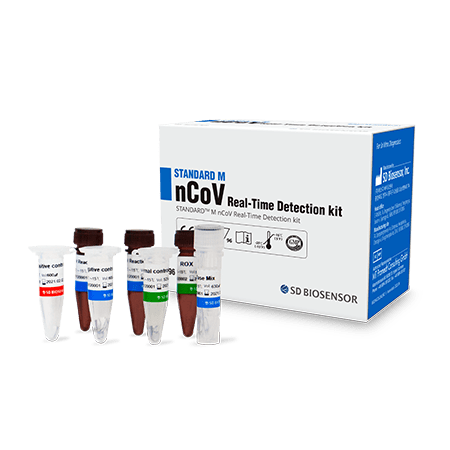
KOREA MFDS Approved  STANDARD M nCoV Real-Time Detection kit is used for rapid identification and detection of novel coronavirus (2019-nCoV) nucleic acids in human nasopharyngeal swabs and throat swab samples.
STANDARD M nCoV Real-Time Detection kit is used for rapid identification and detection of novel coronavirus (2019-nCoV) nucleic acids in human nasopharyngeal swabs and throat swab samples.
- One tube reaction for identification and detection of 2019-nCoV
- One-step Real-Time RT-PCR
- Provide all reagents required for PCR
- Designed according to “WHO interim guidance for laboratory testing for 2019 novel coronavirus (2019-nCoV) in humans”
- nCoV primers/probes ORF1ab (RdRp) gene, E gene
- Provide Internal controls
-
WHO EUL KOREA MFDS Approved TGA Approved
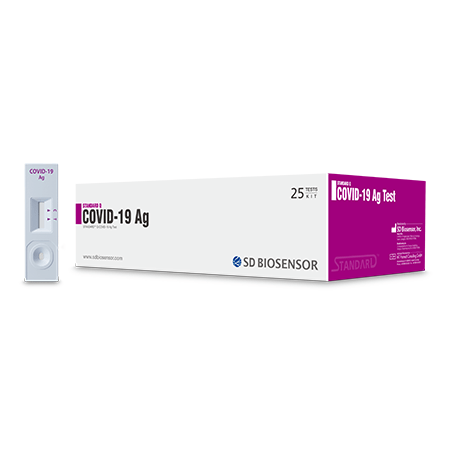
STANDARD Q COVID-19 Ag Test is a rapid chromatographic immunoassay for the qualitative detection of specific antigens to SARS-CoV-2 present in human nasopharynx.
- Fast results within 15~30 mins
- Easy to use
- Specimen : Nasopharyngeal swab
- All necessary reagents provided & no equipment needed
-
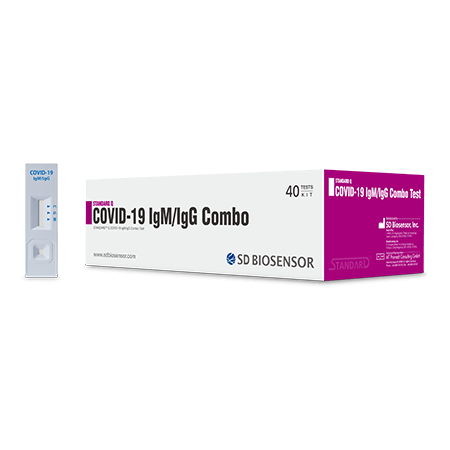
This kit is a rapid immunochromatography test designed for the qualitative presumptive detection of specific IgM and IgG to 2019 novel coronavirus (nCoV) in humoral fluid.
STANDARD Q COVID-19 IgM/IgG Combo Test Kit is a rapid immunochromatography test designed for the qualitative presumptive detection of specific IgM and IgG to SARS-CoV-2 in humoral fluid.
- Rapid testing for SARS-CoV-2 antibodies within 10~15 minutes
- Specimen : Whole blood (20ul), serum , plasma (10ul)
- Suitable for Point of Care Testing. No need for extra equipment
-
This kit is a rapid immunochromatography test designed for the qualitative presumptive detection of specific IgM and IgG to 2019 novel coronavirus (nCoV) in humoral fluid.
STANDARD Q COVID-19 IgM/IgG Duo Test Kit is a rapid immunochromatography test designed for the qualitative presumptive detection of specific IgM and IgG to SARS-CoV-2 in humoral fluid.
- Rapid testing for SARS-CoV-2 antibodies within 10 minutes
- Just 10ul of specimen : Whole blood, serum , plasma
- Suitable for Point of Care Testing. No need for extra equipment
-
This kit is a rapid chromatographic immunoassay for the qualitative presumptive detection of specific IgM and IgG to 2019 novel coronavirus (nCoV) in humoral fluid.
 KOREA MFDS Approved
KOREA MFDS Approved 
STANDARD Q COVID-19 IgM/IgG Plus Test kit is a rapid chromatographic immunoassay for the qualitative presumptive detection of specific IgM and IgG to 2019 novel coronavirus (nCoV) in humoral fluid.
- Rapid testing for SARS-CoV-2 antibodies within 10-15 minutes
- Specimen : Whole blood (20ul), serum, plasma (10ul)
- Suitable for Point of Care Testing. No meed for extra equipment
-
Quick diagnosis STANDARD Q provides rapid diagnostic products with high sensitivity and specificity through quality control from raw material development to production.