-
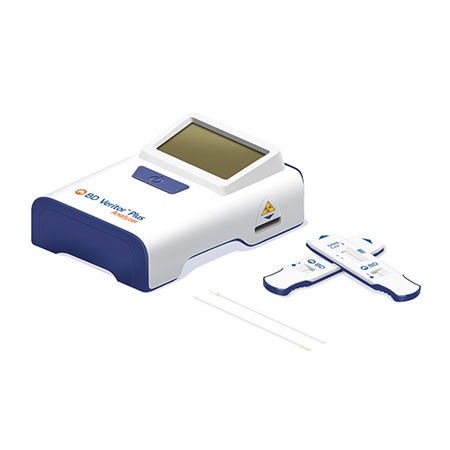
The portable, easy-to-use BD Veritor™ Plus System provides reliable COVID-19 (SARS-CoV-2) results in 15 minutes The BD Veritor™ System for Rapid Detection of SARS-CoV-2 is a chromatographic immunoassay for the direct and qualitative detection of SARS-CoV-2 antigens in nasal swabs from patients with signs and symptoms who are suspected of COVID-19.
-
KOREA MFDS Approved
STANDARD F COVID-19 Ag FIA is the fluorescent immunoassay to detect SARS-CoV-2 infection in human nasopharyngeal swab specimen, identifying existence of SARS-CoV-2 viral nucleoprotein antigens.
-
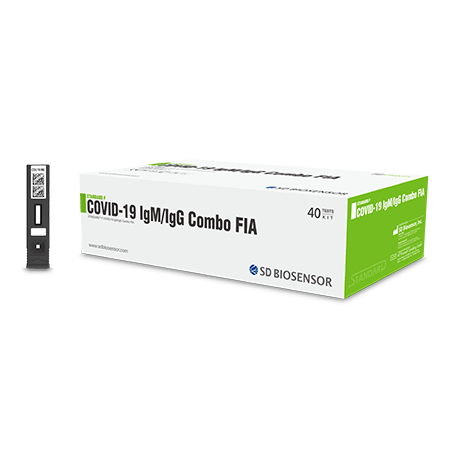
This kit is the fluorescent immunoassay to detect the qualitative presumptive detection of specific IgM and IgG to 2019 novel coronavirus (nCoV) in humoral fluid. KOREA MFDS Approved
STANDARD F COVID-19 IgM/IgG Combo FIA is the fluorescent immunoassay to detect the qualitative presumptive detection of specific IgM and IgG to 2019 novel coronavirus (nCoV) in humoral fluid.
-
This kit is the fluorescent immunoassay to detect the qualitative presumptive detection of specific IgM and IgG to 2019 novel coronavirus (nCoV) in humoral fluid.

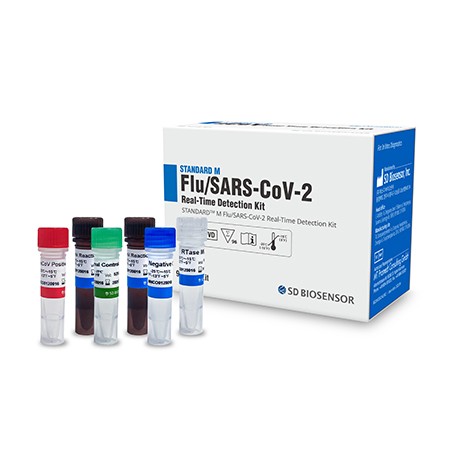
The STANDARD M Flu/SARS-CoV-2 Real-Time Detection Kit is a real-time reverse transcription PCR assay for the simultaneous detection of Flu A, Flu B and SARS-CoV-2 nucleic acids in human nasopharyngeal swab, oropharyngeal swab, and sputum specimens.
- Single tube reaction for detection of SARS-CoV-2, Flu A and Flu B
- One-step Real-Time RT-PCR
- Provide all reagents required for RT-PCR
- Multiple SARS-CoV-2 target genes: ORF1ab and E gene
- Conserved Flu target region: Flu A (Matrix gene), Flu B (NS1 gene)
- Internal control: RNA process control (from NA extraction)
-
 FDA EUA Approved
FDA EUA Approved  KOREA MFDS Approved
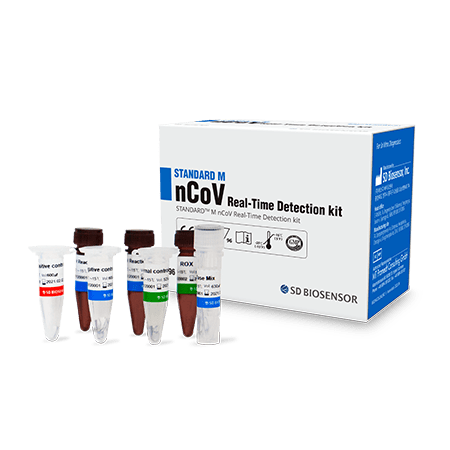
KOREA MFDS Approved  STANDARD M nCoV Real-Time Detection kit is used for rapid identification and detection of novel coronavirus (2019-nCoV) nucleic acids in human nasopharyngeal swabs and throat swab samples.
STANDARD M nCoV Real-Time Detection kit is used for rapid identification and detection of novel coronavirus (2019-nCoV) nucleic acids in human nasopharyngeal swabs and throat swab samples.
- One tube reaction for identification and detection of 2019-nCoV
- One-step Real-Time RT-PCR
- Provide all reagents required for PCR
- Designed according to “WHO interim guidance for laboratory testing for 2019 novel coronavirus (2019-nCoV) in humans”
- nCoV primers/probes ORF1ab (RdRp) gene, E gene
- Provide Internal controls
-
WHO EUL KOREA MFDS Approved TGA Approved
STANDARD Q COVID-19 Ag Test is a rapid chromatographic immunoassay for the qualitative detection of specific antigens to SARS-CoV-2 present in human nasopharynx.
- Fast results within 15~30 mins
- Easy to use
- Specimen : Nasopharyngeal swab
- All necessary reagents provided & no equipment needed
-
This kit is a rapid immunochromatography test designed for the qualitative presumptive detection of specific IgM and IgG to 2019 novel coronavirus (nCoV) in humoral fluid.
STANDARD Q COVID-19 IgM/IgG Combo Test Kit is a rapid immunochromatography test designed for the qualitative presumptive detection of specific IgM and IgG to SARS-CoV-2 in humoral fluid.
-
This kit is a rapid immunochromatography test designed for the qualitative presumptive detection of specific IgM and IgG to 2019 novel coronavirus (nCoV) in humoral fluid.
STANDARD Q COVID-19 IgM/IgG Duo Test Kit is a rapid immunochromatography test designed for the qualitative presumptive detection of specific IgM and IgG to SARS-CoV-2 in humoral fluid.
-
This kit is a rapid chromatographic immunoassay for the qualitative presumptive detection of specific IgM and IgG to 2019 novel coronavirus (nCoV) in humoral fluid.
 KOREA MFDS Approved
KOREA MFDS Approved 
STANDARD Q COVID-19 IgM/IgG Plus Test kit is a rapid chromatographic immunoassay for the qualitative presumptive detection of specific IgM and IgG to 2019 novel coronavirus (nCoV) in humoral fluid.
- Rapid testing for SARS-CoV-2 antibodies within 10-15 minutes
- Specimen : Whole blood (20ul), serum, plasma (10ul)
- Suitable for Point of Care Testing. No meed for extra equipment
-
The NEW Xpert Xpress CoV-2 plus test provides: · Fast and accurate results in as early as 20 minutes* · Three gene targets for SARS-CoV-2 as well as an optimization of N2 probes to enable more reliable virus detection · Rapid sample-to-answer testing with actionable results from a single sample
-
• Robust design with three distinct gene targets for SARS-CoV-2: N2, E, RdRP. • Accurate detection and differentiation of SARS-CoV-2, Flu A, Flu B, and RSV. Results for SARS-CoV-2 in as little as 25 minutes. • Actionable results from a single sample with less than one minute of hands-on time. • Standardization of results between the central lab and near-patient testing sites.
-
Cepheid has developed an automated molecular test for the qualitative detection of SARS-CoV-2, the virus that causes COVID-19. The test leverages the design principles of our current Xpert® Xpress Flu/RSV cartridge technology, in which multiple regions of the viral genome are targeted. The test can provide rapid detection of the current pandemic coronavirus SARS-CoV-2 in approximately 45 minutes with less than a minute of hands on time to prepare the sample. Xpert® Xpress SARS-CoV-2 can be utilized in multiple settings where actionable test results are needed to make informed treatment decisions quickly. The test delivers point-of-care results with the same level of performance seen in reference labs. The test is designed for use on Cepheid’s GeneXpert® Systems, which have a worldwide footprint of more than 23,000 placements.
-
• Detection and differentiation of flu, RSV, and COVID-19 which all present with similar symptoms • Actionable detection of SARS-CoV-2, Flu A, Flu B, and RSV in as little as 25 minutes^ • Actionable on-demand results with 1 sample collection • Optimizes GeneXpert® System module capacity by combining 2 Xpress tests into 1 • Standardization of results between the central lab and near patient testing sites