Ryvex is an authorized distributor of Hardy Diagnostics, a company that manufactures culture media, and rapid identification kits for microbiological testing in clinical, research, and industrial laboratories.
-
The WalkAway plus System delivers gold-standard accuracy for microorganism identification and susceptibility testing. This system delivers accurate emerging resistance detection for the toughest pathogens, including VISA, VRSA, and MRSA.
-
The autoSCAN-4 System processes panels in seconds, simplifying identification and antibiotic susceptibility testing (ID/AST) while standardizing results. Over 30 years of exceptional instrument reliability and our conventional panel technology – with the fewest FDA limitations in automated ID/AST – make the autoSCAN-4 an excellent supplemental system for difficult organisms or as a primary instrument for low-volume usage.
-
As the foundation of our identification and antibiotic susceptibility testing (ID/AST) products, Conventional panels deliver accuracy, choice, and flexibility. With the fewest FDA limitations of leading automated ID/AST systems, use of conventional panels reduces supplemental testing costs and reporting delays associated with rapid only systems.
- Direct minimum inhibitory concentration (MIC) susceptibility methodology helps ensure detection of emerging and low-level resistance, while combo ID/AST formats provide creative workflow solutions.
- Our exclusive, PROMPT™ inoculation method with 4-hour stability reduces inoculum preparation time by over 40% when compared to turbidity methods.
-
Healthcare professionals are faced with a variety of significant fastidious pathogens where resistance to an extensive number of antibiotics is rapidly on the rise. This can lead to complications ranging from pediatric ear infections to life-threatening conditions such as meningitis or sepsis. For appropriate antimicrobial therapy, accurate minimum inhibitory concentration (MIC) testing should be assessed for all significant isolates.
-
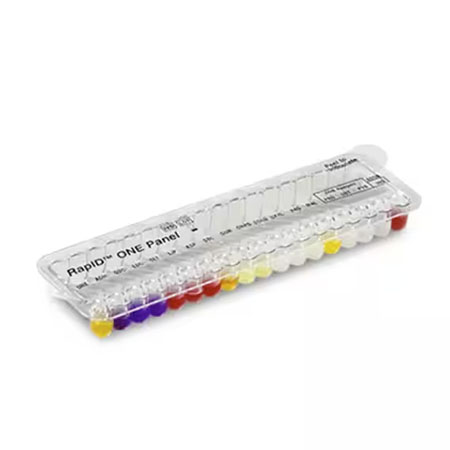
Specialty ID panels provide same-day identification of yeast, Haemophilus/Neisseria, and anaerobic bacteria. The technology tests for the presence of pre-formed enzymes used by the organism to metabolize various substrates.
-
A definitive identification (ID) in as little as 2.5 hours coupled with your hospital antibiogram provides rapid empiric therapy guidance. A good choice for common, high volume, gram-negative organisms – MicroScan Rapid panels achieve same day turnaround time for routine isolates.
-
Organisms possessing Extended-Spectrum Beta-Lactamases (ES?L) may be clinically resistant to therapy with penicillins, cephalosporins, and aztreonam, despite apparent in vitro susceptibility to some of these agents. The incidence of these strains is on the rise and is a significant health concern in some regions. Because of this, the screening and confirmation of ES?L-producing strains is recommended by all major national guidelines.
-
Dehydrated Culture Media is manufactured by Hardy Diagnostics in its ISO 13485 facility. CRITERION™ features: convenient hand grip – stackable containers, four sizes available – 2 liter mylar zip-bag, 500gm, 2kg, 10kg, custom and bulk manufacturing – to your specifications, unparalleled performance – laboratory tested, competitive pricing.
-
-
-
BD supplies more than 400 different BD Difco™ and BD BBL™ brand media formulations and ingredients in dehydrated form for the convenience of the laboratorian and bioprocessing specialist. Quality control The individual ingredients and final dehydrated product are carefully quality controlled to assure consistent, high-quality performance and obviate the need for media to be prepared in the laboratory from raw materials. Sizes Dehydrated culture media are offered in 100-g and 500-g bottles, and 2-kg, 5-lb, 25-lb, 10-kg and 450-g containers. BD™ Select APS TSB Manufactured in an Animal Origin-free, Antibiotic-free facility. Mycoplasma Testing upon release. Endotoxin Testing upon release
-
Since 1935, BD BBL™ prepared media have brought high quality and high performance to the microbiology laboratory. Along with BD Difco™ Laboratories products, BD prepared media are today backed by a combined 180 years of experience in product development, manufacturing and troubleshooting.
-
BD BBL™ Isolator Pack plated media, in gamma-irradiated packs, work with isolator systems. To use the product, place the wrapped package of plates in the isolator before running the vaporized hydrogen peroxide decontamination cycle. When the isolator aeration cycle has finished, remove the two outermost wrappings, and then remove the sterile inner wrapping. Sterility The product is validated for performance and sterility according to Association for the Advancement of Medical Instrumentation (AAMI) guidelines. Transport bag The product includes an inner bag you can use to transport plates from your critical environment. Available media Media include D/E Neutralizing agar, Sabouraud Dextrose Agar with Lecithin and Polysorbate 80, and Trypticase™ Soy Agar with or without lecithin and polysorbate 80. Plate types Available plate types include the BD RODAC™ and RODAC Snap Lid plates for personnel and surface sampling, and BD Finger Dab™ (100mm and 150mm style) plates for sampling of gloved hands.
-
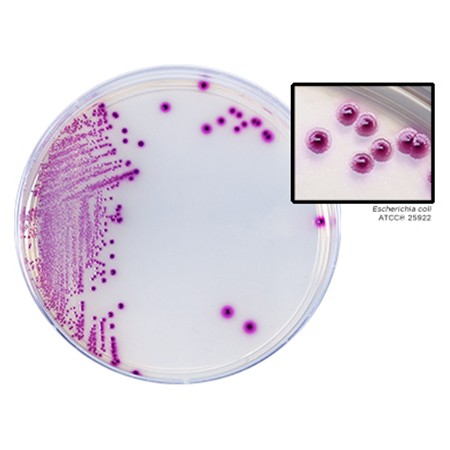
BD BBL™ prepared plated media enable the isolation of microorganisms from samples or specimens. Ready for immediate use, they are routinely available in several types of Petri-style dishes, containing a variety of media. BD Stacker™ dishes and BD Stacker™ I Plate™ dishes These 100-mm x 15-mm–style dishes interlock, minimizing the hazard of sliding dishes. BD Stacker dishes contain one medium. BD Stacker I Plate dishes with two sections contain one or two media. Quad plate 100-mm x 15-mm–style dishes These dishes feature four sections that each contain a different media formulation. BD RODAC™ and BD RODAC™ Snap Lid plates These plates are for surface sampling of microbial contamination, featuring a base with inward-sloping walls and a convex bottom designed to keep the agar bed in place during transit and use. The BD RODAC Snap Lid plate features a unique snap-locking system to provide additional security during sampling and transport.
-
BD BBL™ prepared tubed media enables the isolation and identification of microorganisms. Tubed media are ideal for cultures requiring prolonged incubation, since many can be incubated with tightly closed caps to prevent dehydration and subsequent inhibition of growth. Offering a wide variety of sizes, we can manufacture your individual culture medium in the size your application demands. Eliminated contamination risk Special long-necked, rubber-lined screw caps eliminate danger of contamination. Easy inoculation Larger-sized tubes with wider diameters permit easy inoculation and media handling. Moisture loss prevention Tubes are borosilicate glass with black screw caps securely fitted to prevent loss of moisture. Displacement resistance Most tubes contain a special resilient rubber liner, which resists displacement even during autoclaving.
-
BD BBL™ Sterile Pack plated media are in gamma-irradiated packs, validated to performance and sterility according to Association for the Advancement of Medical Instrumentation (AAMI) guidelines. They also feature outer DuPont™ Tyvek® polyethylene wrapping that minimizes the risk of contamination, provides a fiber-free bacterial barrier and allows gas exchange. To use this media, remove the outer wrapping in the noncritical environment, and then remove the sterile inner wrapping in the clean area. The included bag may be used to transport plates from your critical environment. This media must be stored at a temperature of 2°C to 8°C. Extensive availability BD BBL Sterile Pack plated media are available in many formulations and plate types. Personnel and surface sampling BD RODAC™ and BD RODAC™ Snap Lid plates support personnel and surface sampling. Air sampling BD BBL Sterile Pack settle plates provide active and passive air sampling. Gloved hands sampling BD Finger Dab™ plates allow sampling of gloved hands.