-
Prereduced anaerobic culture media packaged in foil pouches that are flushed with oxygen-free gas. Wide variety of packaging combinations are offered: mono-plates, bi-plates and primary setup combinations. Competitive pricing.
-
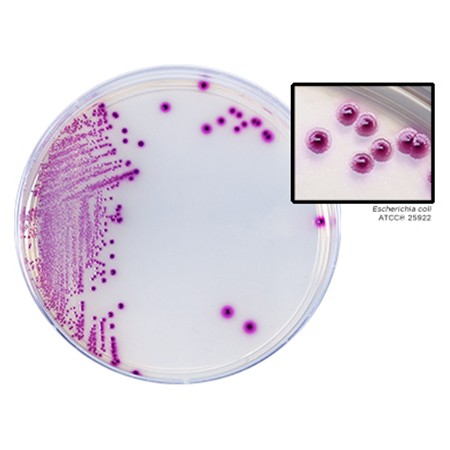
A primary screening medium for blood, sputum, or urine samples for the simultaneous detection and differentiation of gram-negative bacteria that produce carbapenemase. It can be used as a cost effective screening method.
-
HardyCHROM™ MRSA is a selective and differential chromogenic medium for the qualitative direct detection of nasal colonization by methicillin-resistant Staphylococcus aureus (MRSA). Not intended to diagnose infection or guide therapy.
-
Large selection of control organisms derived from ATCC for performing quality control testing in your laboratory.
-
- Detects the presence of viable Mycobacterium tuberculosis
- Performs susceptibility testing simultaneously and within the same procedure.
- Detects resistance to both isoniazid and rifampicin; the two most commonly used drugs for therapy.
- Uses liquid culture for accelerated growth.
- Utilizes the TB cording phenomenon for identification, which is easily viewed with an inverted microscope.
- Detects susceptible, mono-resistant, and multi-drug resistant (MDR) TB usually within a 5 to 10 day incubation period.
- Can be used to monitor patients on therapy. Detects only living organisms.
- Cost effective and labor saving
- Ready-to-use antibiotics available in an easy-to-dissolve tablet. No measuring, weighing, or mixing needed.
- All vials and reagents are color coded to simplify and error-proof the procedure.
- Unique protective flexible silicone sealing lid for increased safety. Tray remains permanently sealed throughout the entire incubation and examination procedure. Safety lid can be easily pierced with a needle/syringe if sub-culturing or rapid specification is necessary.
-
Hardy Diagnostics offers a complete selection of products for parasitology testing and transport of specimens.
-
For the reliable detection of Group B Strep (Streptococcus agalactiae) in pregnant women. Recently recommended by the CDC for testing women in the 35th to 37th week of pregnancy. Found to be 100% accurate for hemolytic strains.
-
Susceptibility Testing HardyDisk™ ASTs are impregnated paper disks used for Antimicrobial Susceptibility Testing (AST); also known as disk diffusion or Kirby-Bauer testing. Each cartridge contains 50 disks that are spring loaded for smooth and reliable delivery from your BD dispenser.
-
TransPRO™ is a liquid-based multi-purpose bacterial collection and transport system utilizing the new HydraFlock™ swab technology in a liquid amies medium